Our selection for your ideas –
Materials for your jewelry dreams.
Choose your perfect foundation
With us, you have the freedom to choose from a wide variety of materials for your jewelry pieces. Every design is discussed with us in advance. For rings, the width and thickness of the material are flexible, allowing us to meet your individual wishes and requirements.
Our material selection includes both single-color and multi-color options. For multi-color materials, countless combinations are available. You can design your material lengthwise or crosswise, work with inlays, or select an inside/outside color combination – exactly according to your ideas and the design of your jewelry.
Our team is happy to support you in selecting and realizing your ideas, ensuring that your jewelry is unique and made precisely to your wishes. Please note that in consultations we may also advise against certain options. After all, nobody wants to wear a wedding ring that is too thin or has sharp edges.
Color & Profile Selection
Materials
We offer high-quality alloys, including:
-
14k yellow gold (585)
-
14k white gold (585)
-
14k red gold (585)
-
18k yellow gold (750)
-
18k white gold (750, with 12% palladium content)
-
18k red gold (750)
-
18k rose gold (750)
-
21.6k yellow gold (900)
-
Platinum (950 with 5% tungsten content)
We source all of our gold alloys exclusively from the secondary cycle, meaning they come from recycled material. Since gold mining – even under Fairtrade conditions – inevitably causes environmental damage, we and our suppliers choose to avoid mined gold entirely. We therefore appreciate it when our customers bring in more gold and silver for recycling.
Surface Finishes
-
High polish: Classic, shiny look
-
Silk matte (lengthwise/crosswise): A soft, matte finish with a subtle shimmer depending on the direction of processing
-
Ice matte: A refined, frosty surface effect
-
Sandblasted: A textured, matte surface with a unique appearance
-
Hammered: A handcrafted look with various structural options
-
Milled: Precisely worked surface with fine, individual patterns (e.g., double or Saturn wave)
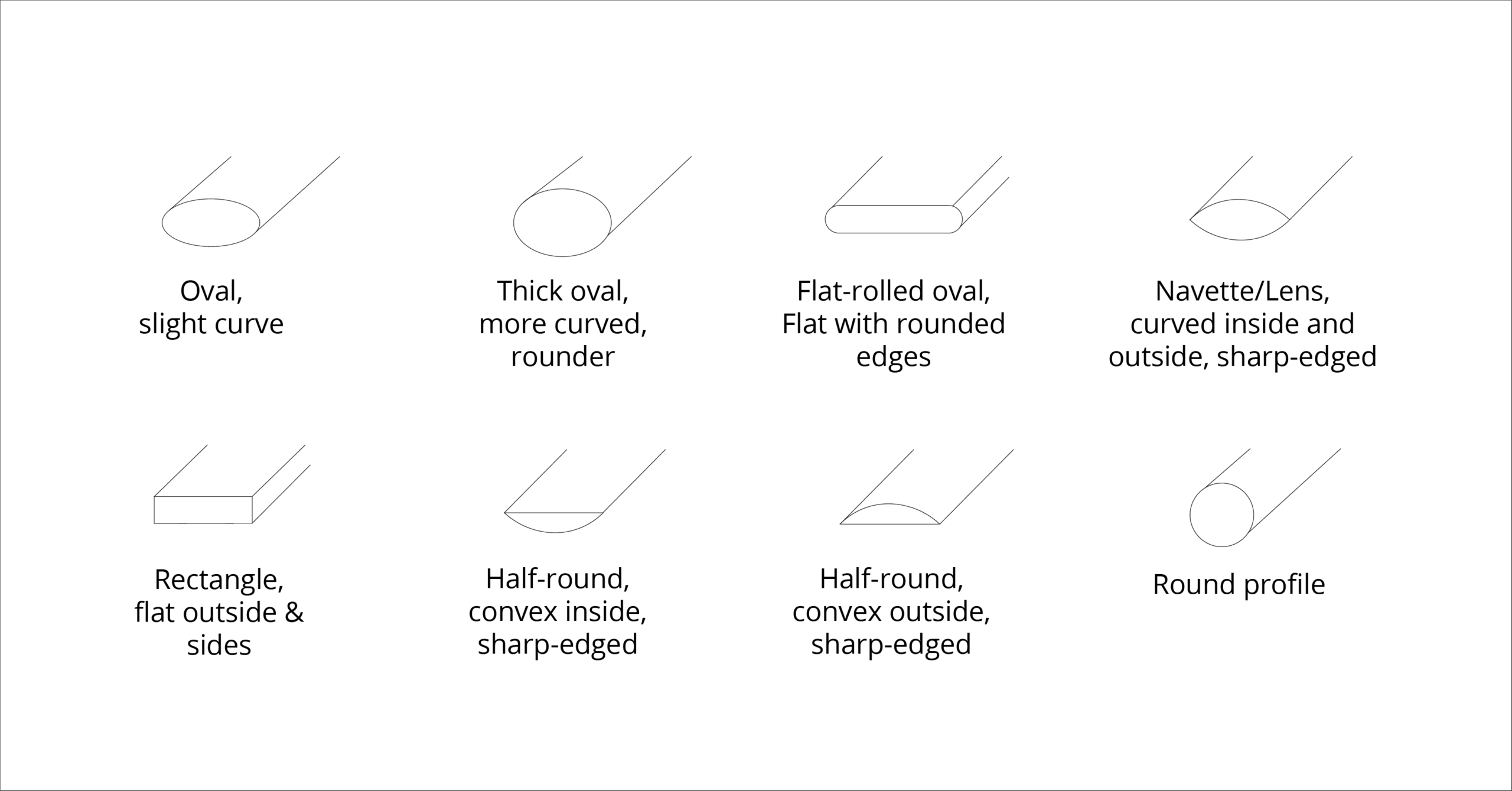
Ring Cross-Sections
In addition to choosing the material, you can also determine the cross-section of your ring. Whether rectangular, oval, or half-round – the shape gives your ring a distinctive character and strongly influences its overall look. Choose the cross-section that is most comfortable to wear, especially with the thought that the ring will sit on your finger for decades. This is why 95% of all wedding rings are made with an oval cross-section.
Gold alloys
Why is gold alloyed?
Pure gold (fine gold) is rarely used due to its very low hardness. To improve its wearability, it is alloyed – that is, mixed with other metals such as silver, copper, palladium, titanium, or gallium in varying proportions.
The numbers 990, 900, 750, 585, and 333 indicate the percentage of fine gold in the alloy.
Why is 18k (750) gold better?
The main advantages of 18k gold (formerly referred to as 18 carat) are its beautiful color and noble character (it never tarnishes under normal, everyday use).
We goldsmiths consider alloys of 18k and higher to be resistant. Some goldsmiths no longer even refer to alloys below 18k as real gold… and they are right!
Gold Alloy Table
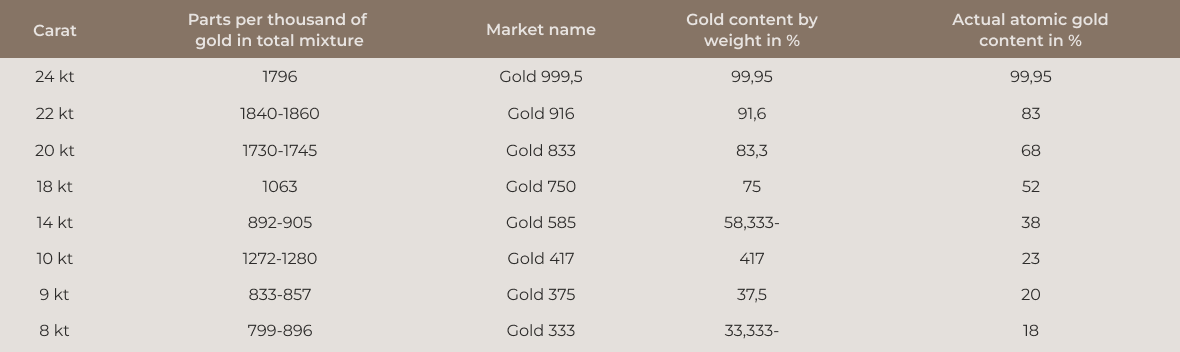
With a gold content of 75%, there are in fact “only” about 52% gold atoms present. The density of gold, at 19.3 g/cm³, is roughly twice as high as that of the additional metals.
That this 50% (or more) is clearly an advantage can easily be explained by the properties of pure gold:
Gold, like platinum and its related metals, is a noble metal of the highest order and can therefore protect base metals from oxidation or sulfidation merely by its presence – but only one base atom per gold atom. If less gold is present than alloy metals (e.g. 14k / 585 gold with 38% content), some base atoms “float” between the gold atoms unprotected and may oxidize, sulfidize, corrode (creating stress within the gold), and in the worst case, even after years of normal wear, lead to allergies or fractures.
The common prejudice that 14k (585) gold is much harder than 18k (750) gold is no longer valid with modern manufacturing processes and alloys. Even the traditional 18k alloy is only marginally softer.
Unfortunately, some jewelers, so-called “sales specialists,” continue to spread this “argument.” The reason is as simple as it is unflattering: 14k gold offers a higher profit margin… one of many reasons why goldsmiths do not like to be called jewelers.
White Gold
There is no such thing as naturally white gold. Gold is naturally yellow and was only made as white as possible from 1921 onward by adding alloys such as nickel, in order to offer a more affordable alternative to platinum. Initially, this whitening was achieved with high amounts of nickel.
Since gold may no longer be alloyed with unlimited amounts of this metal (about 30% of the population reacts allergically to nickel), our white gold is exclusively whitened with palladium, a metal from the platinum group. Palladium is significantly more expensive but causes skin reactions only in extremely rare cases.
Platinum
Unparalleled Color
White, yet not white – a mysterious dark shimmer surrounds platinum like an aura, drawing every gaze.
Rarity
Platinum is about 30 times rarer than gold and extremely difficult to mine, as it successfully resists nearly all chemicals. Platin ist circa 30-mal seltener als Gold und nur sehr schwer zu gewinnen, da es sich gegen nahezu alle Chemikalien mit Erfolg zur Wehr setzt.
Purity
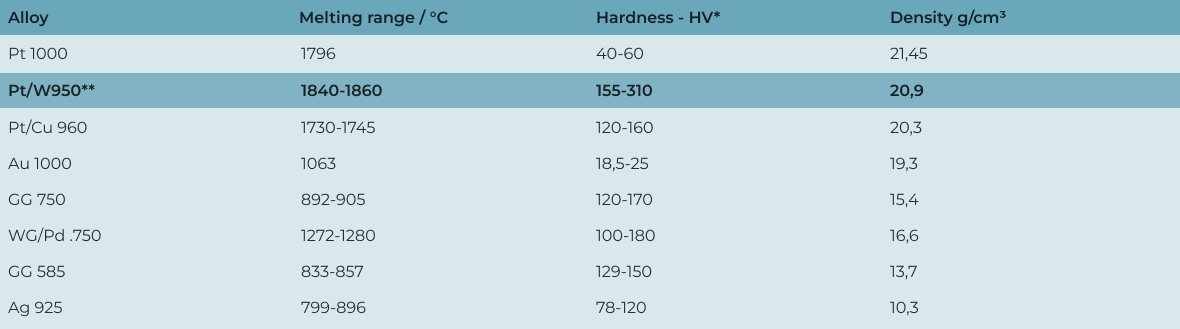
Because of these properties, platinum does not need to be heavily alloyed, unlike gold, in order to achieve wearability. Just 4–5% additional metals are sufficient (960 or 950 platinum), which means its character is hardly altered.
Compatibility
Platinum is absolutely hypoallergenic, meaning skin reactions are not possible. There would be much more to say, most of it relating to its processing rather than wearing it as jewelry.
Extremely low thermal expansion
-
Not prone to hot-cracking (unlike gold and silver)
-
Very poor heat conductor and therefore weldable (ever held 1700°C without protection just 2 cm from your fingers?)
Eternity
- No natural acid can attack or dissolve it
- No natural flame can melt it
- No discoloration, even when alloyed
- Almost no wear (due to its high tenacity, ductility, and hardness) in daily use
- Platinum jewelry does not lose mass… it is eternal
Weight
How can something so small be so heavy? With a density of 21.45 g/cm³, platinum is the third heaviest of all metals – only osmium and iridium, both platinum group metals, are heavier. Even radioactive heavy metals cannot compare. A cube with an edge length of 16 cm weighs about as much as an adult man – 88 kg! This translates into a very pleasant wearing experience for wedding rings (or jewelry): noticeable but not bothersome, always reminding you of the person who wears the “other” ring.
Other Applications
-
Laboratory equipment for medicine and chemistry
-
Electrical engineering: wires, contacts, anodes for electroplating baths
-
Standards of measurement (the original meter and kilogram were made of Pt/Ir)
-
Catalysts in chemistry
-
Extremely wear-resistant spinnerets for synthetic fiber production
-
Core material in medical devices such as pacemakers
-
…and much more
The History of Platinum
Platinum is a relatively “new” metal, as its very high melting point of 1,774°C meant it could not be melted by natural fire. It was only with the development of pure oxygen in 1772 that a sufficiently high flame temperature could be reached. However, the history of this most noble of noble metals goes back to the 3rd millennium BC. The Egyptians incorporated small amounts into jewelry, probably mistaking it for silver. At the end of the 16th century, the French chemist Lavoisier tried to melt platinum using a magnifying glass with a focal length of 3 meters and a lens 1.2 meters in diameter – without success.
In the 17th century, platinum first attracted more attention in the Spanish colonies such as Ecuador, albeit in a negative sense. Since platinum has almost the same density as gold (Pt: 21.45 g/cm³ vs. Au: 19.3 g/cm³), it was used as a core in gold coins to “stretch” them. The Spanish government even ordered large quantities of platinum to be sunk in the sea to stop this practice. From this period also comes the name platinum, meaning “little silver”, since people simply didn’t know what to do with it. Some gold prospectors even threw it back into rivers to “mature”, believing it to be unripe gold because of its color but unusually high density. In 1751, platinum was finally recognized as a precious metal.
Around 1780, King Louis XVI of France decreed that only the king himself was allowed to own platinum. In 1795, the original meter and kilogram were made from a platinum-iridium alloy, as it was perfectly suited due to its properties. In 1856, the first hydrogen-oxygen platinum melt was created, making it possible to process platinum in “larger” quantities. In the following decades, platinum celebrated a triumph through aristocratic and royal houses. Among other things, the two largest diamonds in the world were set in platinum: Cullinan I in the scepter and Cullinan II in the crown of Elizabeth Bowes-Lyon (mother of Queen Elizabeth II).
Request your
appointment now!
a unique memory.
